MammaTyper® is a new tool for subtyping breast cancer.
READ MORE +ABOUT US
Cerca Biotech
Cerca Biotech is the European subsidiary of Shuwen Biotech, a highly reputable and innovative molecular and point of care diagnostics organisation. Shuwen and Cerca are passionate about healthcare and are working to create world-leading innovations in the fields of Oncology and Women’s Health.


High-performance test
Quantitative RT-qPCR assay (CE marked IVD)
- Highly reproducible biomarker assessment
- Reliable results through standardized biomarker detection
- Accurate stratification of breast cancer tumors into St Gallen subtypes
Promising clinical utility
Clinical value validated in numerous performance evaluation studies
- Outperforms IHC by accurate Ki-67 determination
- Provides information on patient‘s prognosis
- Accurate subtyping supports treatment decisions
Easy-to-use
Clinical value validated in numerous performance evaluation studies
- Reliable method for any molecular pathology laboratory
- Validated for multiple qPCR instruments*
- From resection or core needle biopsy FFPE sample to result within 6 hours FFPE = formalin fixed paraffin embedded
NEWS
Recent article and news
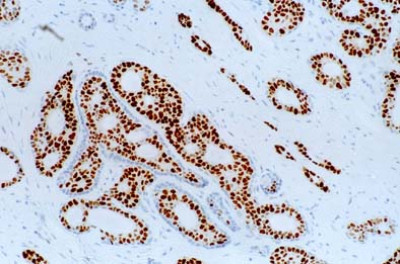
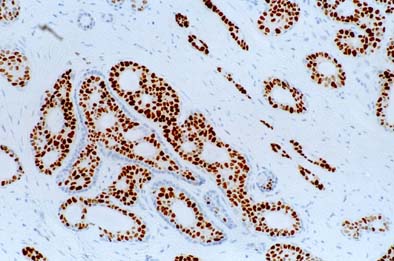
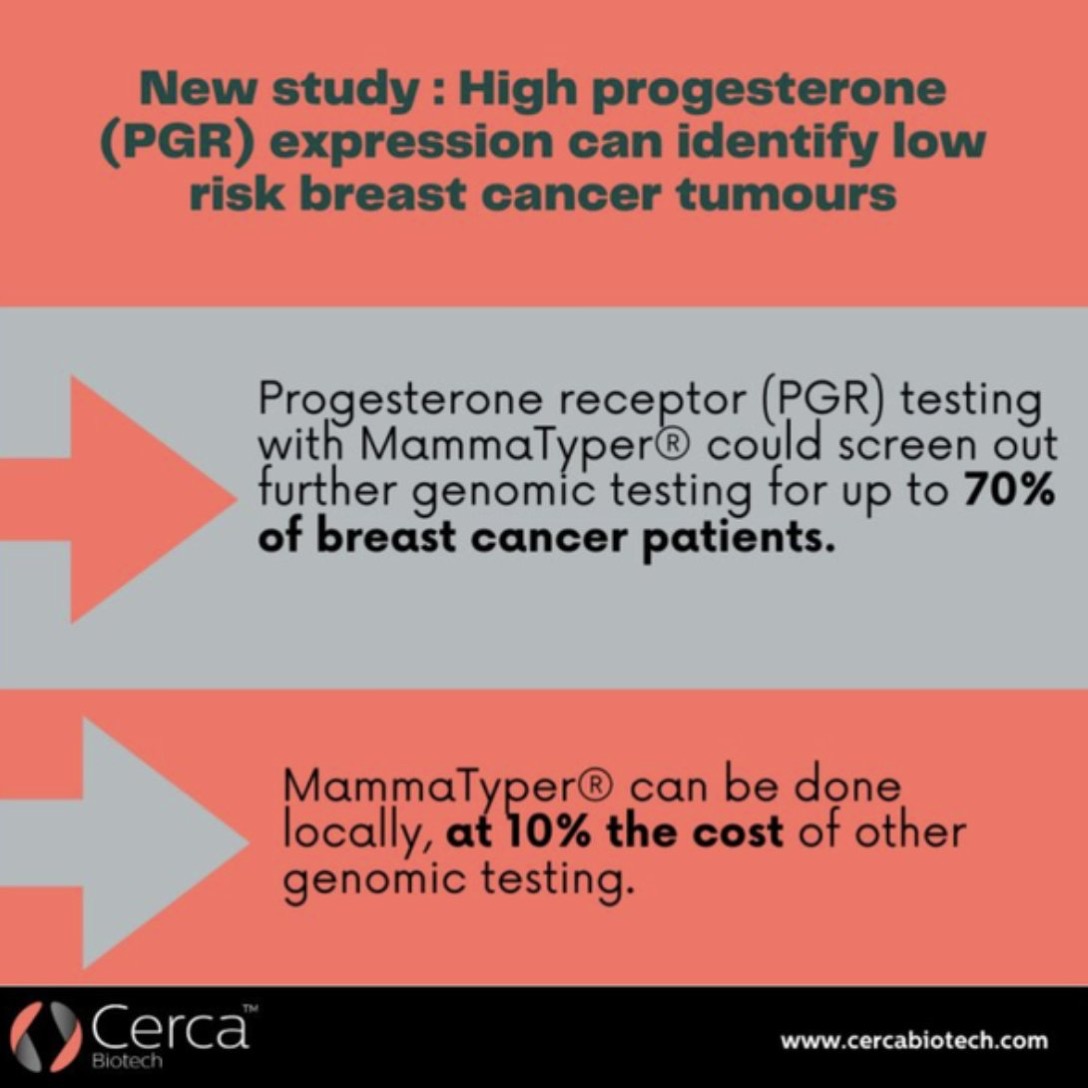
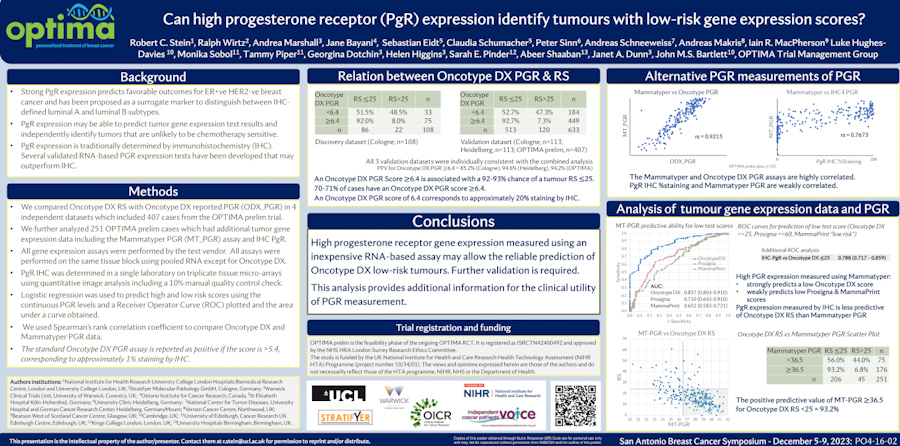
Predicting recurrence with progesterone receptor testing using MammaTyper®
One of our four key goals for clinical validation in 2024 is to demonstrate that progesterone receptor (PR) testing with MammaTyper® could be used for predicting risk of recurrence. In this article, we assess the current role of PR, where IHC testing falls short, and how MammaTyper® could provide a reliable, more accessible option for risk scoring.
PR – the forgotten marker?
In breast cancer testing, progesterone receptor (PR) is sometimes considered the ‘forgotten marker’. Unlike ER or HER2, there are no treatments in current clinical practice linked to the presence or absence of PR. As a res...
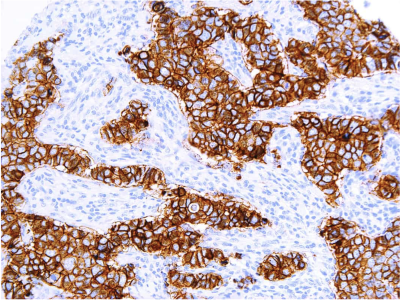
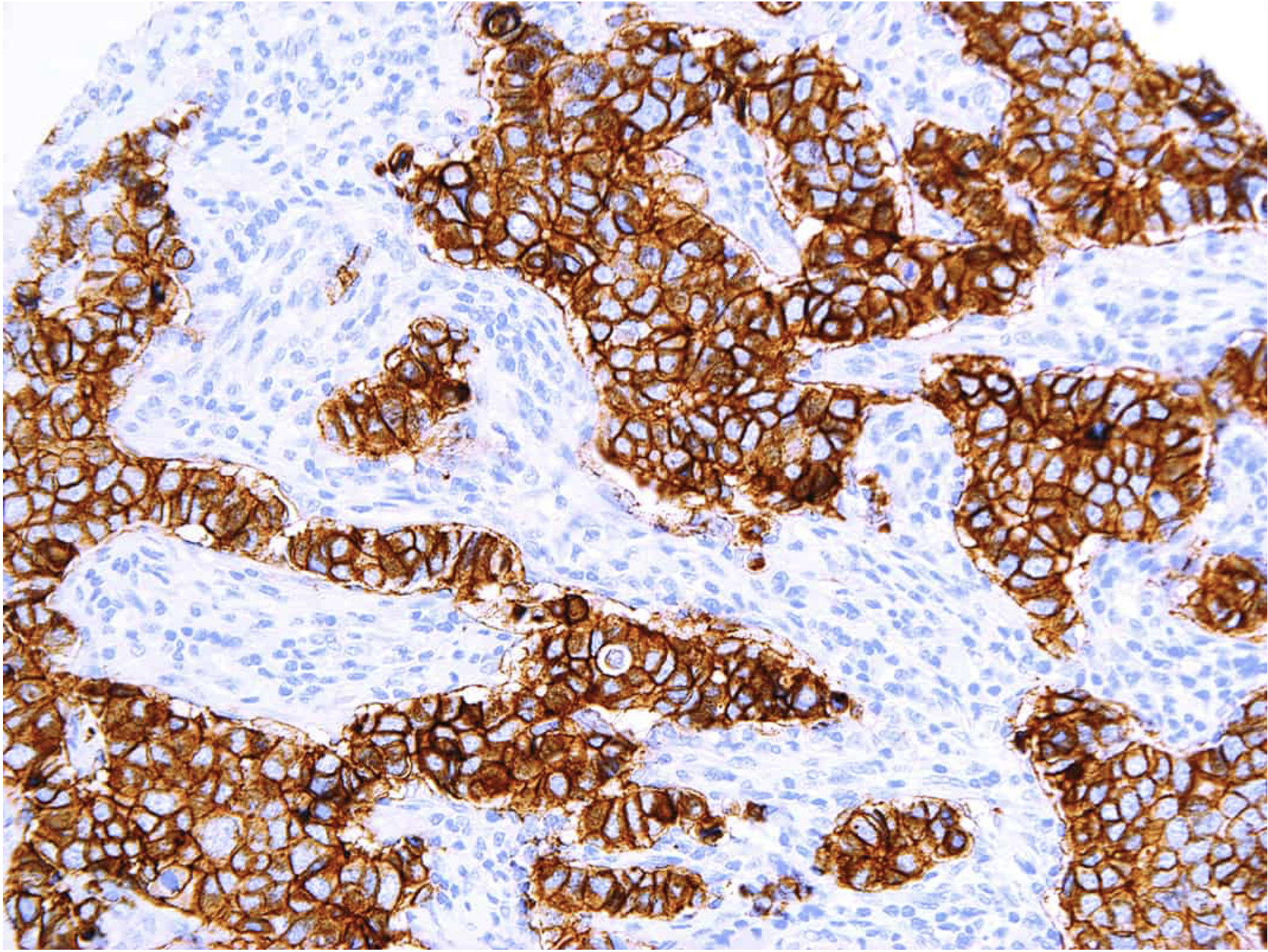
Defining HER2-low using MammaTyper®
One of our four key goals for clinical validation in 2024 is to demonstrate that MammaTyper® is a more reliable way to define HER2-low status. In this article, we assess the importance of HER2-low, where IHC testing falls short, and how MammaTyper® could improve diagnosis and treatment for this group of patients.
Cerca Biotech’s 2023 Highlights
2023 was a stand out year for Cerca Biotech. We doubled our team, added a major partner in Sysmex Europe, and were present at all the major congresses. At the same time, the pharmaceutical world took some big steps forward with defining trials in ADC and CDK 4/6 therapy, both of which have an inherent need for more reliable and reproducible testing for HER2 and Ki67 - which MammaTyper® can provide.
We look back on the work we have done this year around branding and marketing with pride. Our small but massively experienced team has pulled together to put MammaTyper® on the map in a way we could...