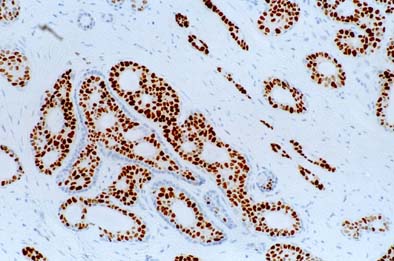
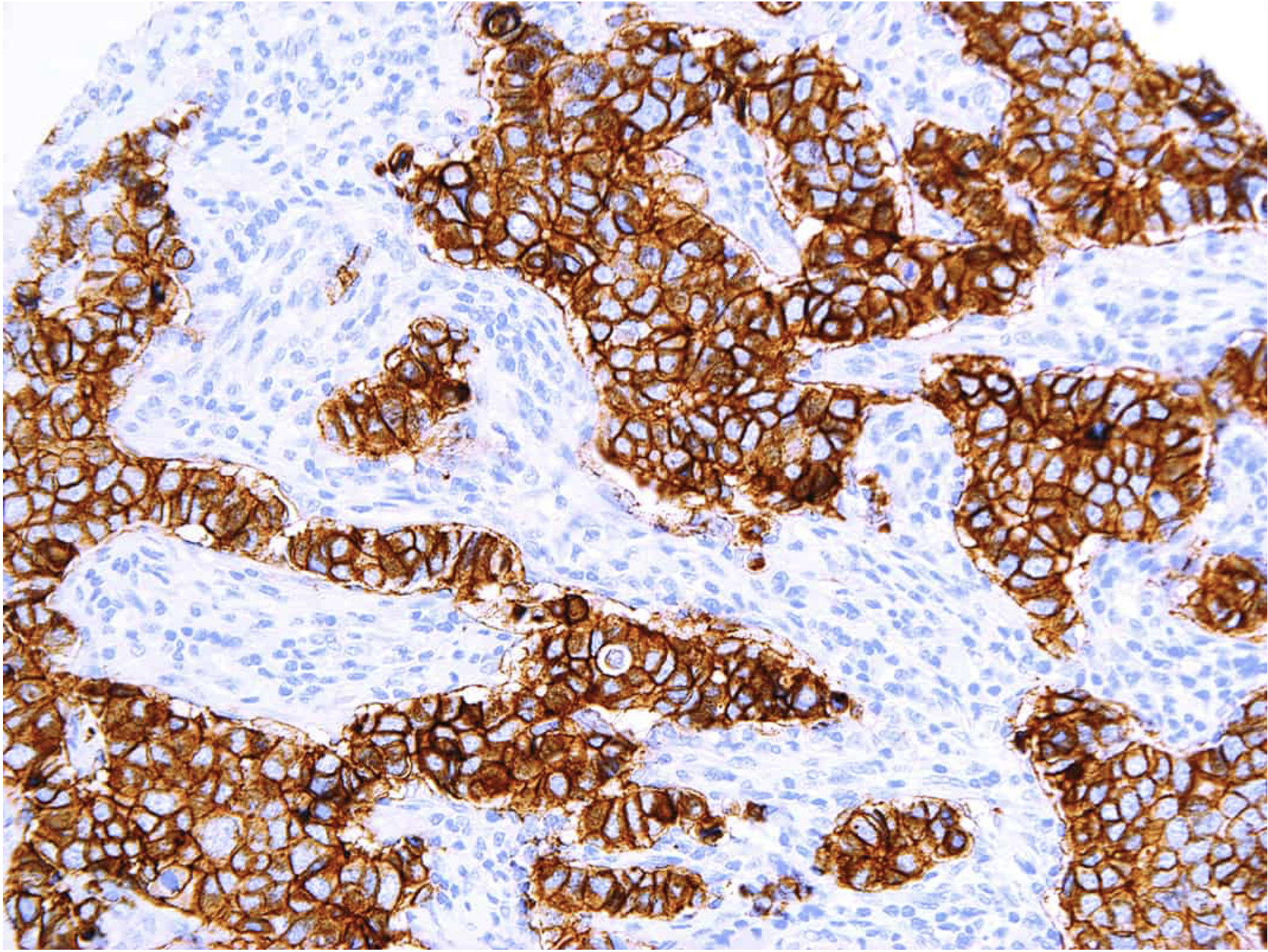
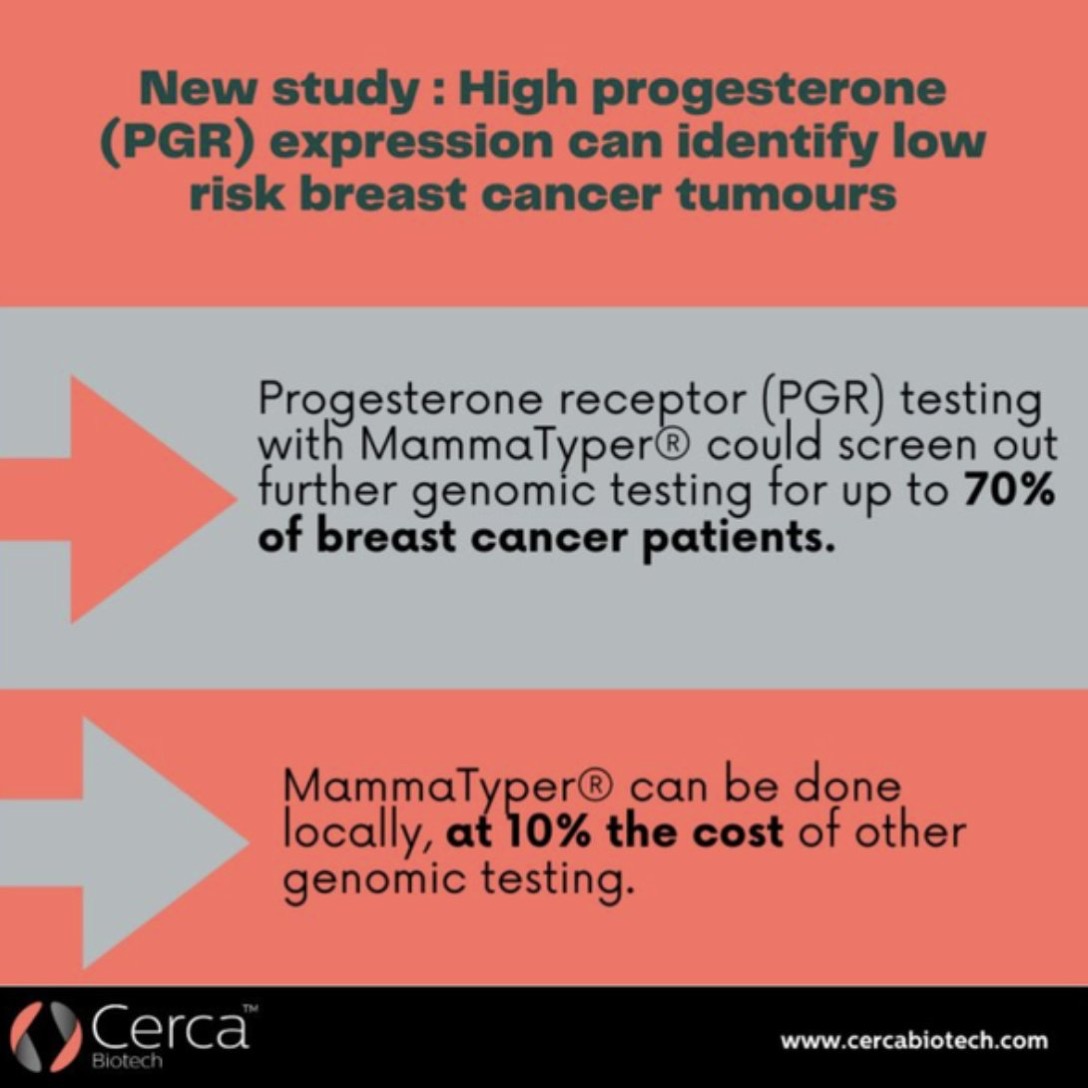
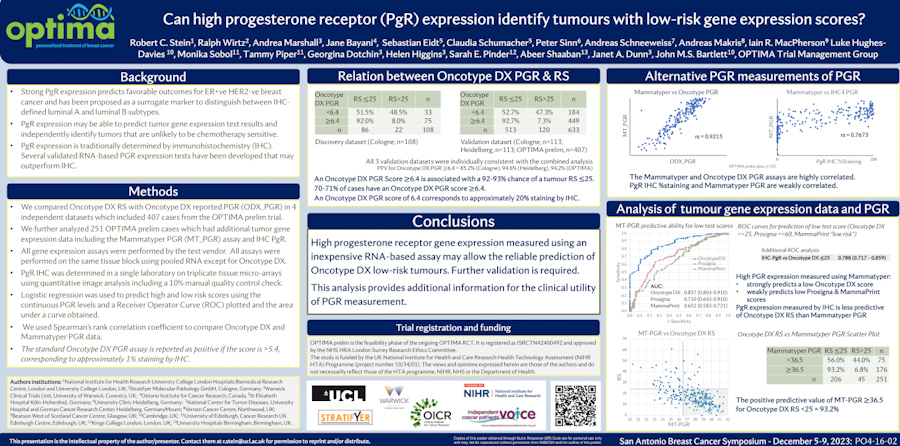
MammaTyper® is a new tool for subtyping breast cancer.
READ MORE +ABOUT US
Cerca Biotech
Cerca Biotech is the European subsidiary of Shuwen Biotech, a highly reputable and innovative molecular and point of care diagnostics organisation. Shuwen and Cerca are passionate about healthcare and are working to create world-leading innovations in the fields of Oncology and Women’s Health.


High-performance test
Quantitative RT-qPCR assay (CE marked IVD)
- Highly reproducible biomarker assessment
- Reliable results through standardized biomarker detection
- Accurate stratification of breast cancer tumors into St Gallen subtypes
Promising clinical utility
Clinical value validated in numerous performance evaluation studies
- Outperforms IHC by accurate Ki-67 determination
- Provides information on patient‘s prognosis
- Accurate subtyping supports treatment decisions
Easy-to-use
Clinical value validated in numerous performance evaluation studies
- Reliable method for any molecular pathology laboratory
- Validated for multiple qPCR instruments*
- From resection or core needle biopsy FFPE sample to result within 6 hours FFPE = formalin fixed paraffin embedded
NEWS
Recent article and news

Expert Roundtable discussion on the current state of breast cancer diagnostics
We gathered four experts to discuss how breast cancer treatment has advanced, whether current diagnostics are up to the task, and how we can move forward in the molecular era for the benefit of breast cancer patients. Our four experts are: ● Prof John Bartlett – retired professor of molecular biology from the University of Edinburgh. ● Dr Liz O’Riordan – retired consultant breast surgeon who has had breast cancer three times. ● Prof Emad Rakha – professor in breast pathology from the University of Nottingham, and honorary breast consultant at Nottingham University Hospital. ● Prof Abeer Shaaban – specialist breast pathologist working at the University of Birmingham and Queen Elizabeth Hospital Birmingham.

Predicting response to neoadjuvant endocrine therapy with Ki67 testing
Ki67 testing could play a vital role in helping tailor treatments for people with ER-positive breast cancers. However, long-standing problems with immunohistochemistry (IHC) are preventing patients from benefitting from this biomarker. In this blog post, we outline the potential of Ki67 in the neoadjuvant setting, and why we need to look at alternatives to IHC.

Using RT-qPCR to identify patients liable to show resistance to endocrine therapy
Endocrine therapy has for decades been one of the key treatment choices for hormone receptor-positive breast cancer. Yet predicting who will and won’t benefit from therapy remains a challenge. In this article, we explore whether gene expression profiling with MammaTyper® may make up for the shortfalls of IHC in this area.